Spectral Filters in the Bullfrog Ear
Notes from seminar given August 13, 2008,
1. Why Spectral Filters?
What
are their selective advantages?
a. Rejection of noise and interference
Example:
•
Presume that the bullfrog
sacculus is a micro-seismic sensor whose function
(adaptive value) lies in its ability to alert the frog to significant
vibrations in the ground—such as the footfalls of predators.
•
The amplitude of
micro-seismic noise on the earth’s surface is very high at frequencies below
about 5 Hz.
•
Signals from
footfalls and other impulsive micro-seismic stimuli in the ground have spectral
peaks at higher frequencies (usually centered about 50 Hz).
•
To detect these,
it would be appropriate to suppress saccular inputs
in the in the frequency range below 5 Hz.
•
Responses to
those inputs could mask or interfere with responses to the vibrations from
predator footfalls.
•
Being
supersensitive to vibration, the bullfrog sacculus is
sensitive to airborne sound as well— responses to which also could mask or
interfere with the signals in the ground.
•
The bullfrog
amphibian papilla responds to the acoustic spectrum for several octaves,
beginning at about 100 Hz.
•
Therefore it would
be appropriate to suppress saccular inputs in the
frequency range above approx. 100Hz..
•
By focusing on
the spectral region between about 5 Hz and about 100 Hz, the bullfrog sacculus would cover most of the spectral energy of
footfalls, while suppressing interference from a wide range of other sources.
b. Spectro-temporal
analysis
Example
•
Separation of
signals from one another is a quintessential part of hearing.
•
The acoustic
input arriving at each ear commonly is a mixture of sounds from many
sources. Each of the two cochleae
carries out an ongoing spectrographic decomposition of its acoustic input, the
human brain then is able to infer (computationally) which spectro-temporal
components belong to a single waveform (from a single source) . . .
•
and to recombine those components to form a perception of
that waveform.*
•
The computations
require spectro-temporal decomposition with high
resolution in both frequency and time.
•
This can be
achieved with filter functions (impulse responses) that are compact in time and
still provide sharp spectral discrimination - - -
•
implying the use
of very steep band edges rather than
very narrow pass-bands for spectral discrimination.
•
By extension, I
presume that non-human listeners employ comparable computations with the same
filter requirements.
* Perception, of course, is a mental
construction. Investigators depend on
reporting from human subjects in order to infer that a particular perception
has occurred. Understanding the linkage
between neural constructions underlying perceptions and perceptions themselves
still seems beyond the reach of natural science. The psychophysicist bridges that gap by
mapping observable physical parameters (e.g., of complex acoustic waveforms) to
reportable perceptions. The neural
algorithms and filter requirements for spectral decomposition in the human
listener have been inferred, strongly, from auditory psychophysics.
2. Examples of acoustic filters in the vertebrate ear
Here are filter functions from gerbil, turtle
and bullfrog ears, observed at common background sound levels - - - 40 decibels and above
a.
Sample filter functions (impulse responses) from gerbil cochlear units, with
spectral peaks at approximately ˝-octave intervals (Lewis, Henry, Yamada, 2002)
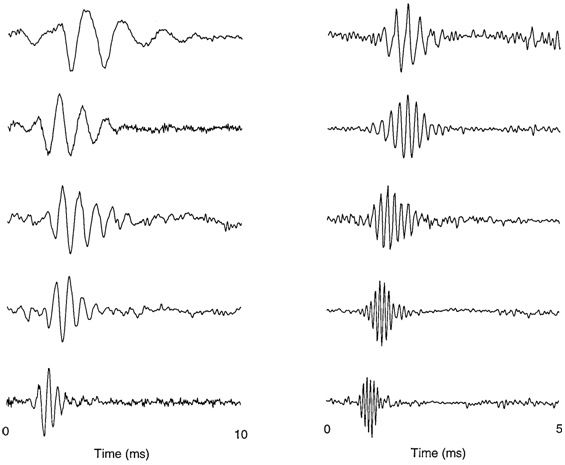
Note: the filter functions on the left
were estimated by REVCOR (1st-order Wiener kernels).
Those on the right were derived
from decomposition of the 2nd-order
Wiener kernels (see
“Saccular
physiology from the outside,” part I).
Amplitude
DFTs (Discrete
Fourier Transforms) of the same gerbil filter functions
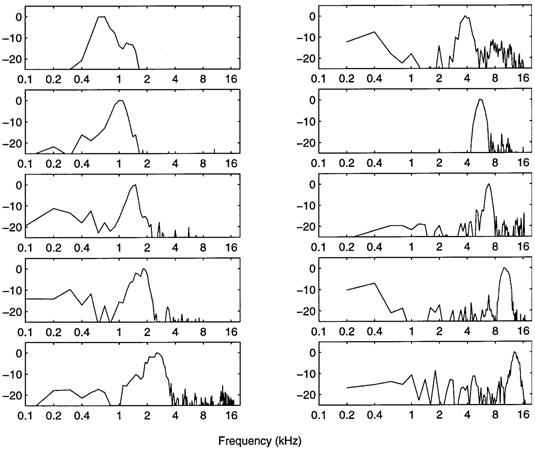
The noisiness of these Discrete Fourier
Transforms reflects the noisiness inherent in the
impulse-response estimation procedure
(reverse correlation of spike occurrences with
broad-band noise stimuli). From the noisy base in each DFT rises the
relatively
smooth tuning peak of the unit.
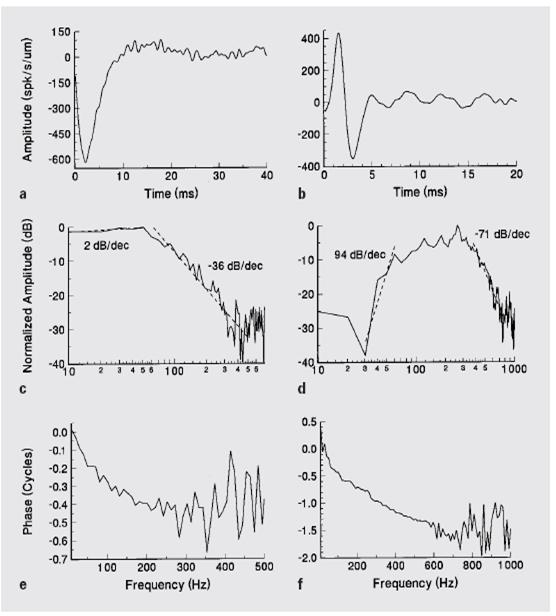
b. How faithfully do the filter functions
represent the actual filters?
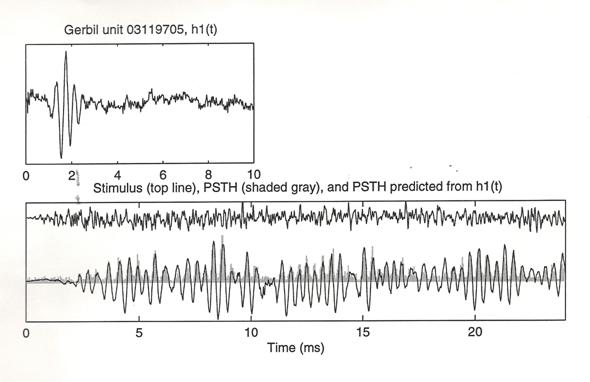
The upper frame shows the
filter function, h1(t). It
was computed for a gerbil cochlear unit by reverse (triggered) correlation
between continuous (non-repeating) noise stimulus and the unit’s spikes. In the lower frame, the top line shows a
different broad-band noise waveform that was presented repeatedly—as a closed-field
airborne sound stimulus to the gerbil’s ear canal. Beneath that, in gray, is the spike-rate response (Peri-Stimilus Time Histogram) of the
same gerbil unit to this same, repeated stimulus. The superimposed solid line is the
spike-rate response predicted from the filter function (the convolution of the
filter function with the repeated waveform).
The filter function clearly predicts the amplitudes and phases of the
positive spike-rate peaks beautifully.
It fails to reflect at all, however, the fact that spike rates cannot be
negative. The strong clipping, imposed
by that fact, can be eliminated
experimentally by adding non-repeating dithering noise to the repeated stimulus
waveform. Even without that, the results
shown here strongly support the fidelity of the filter function in representing
the tuning and timing of this gerbil unit’s response. Furthermore, the filter functions all are compact in time and yield tuning curves with steep band edges.
c. Sample filter functions from the
basilar papilla of the red-eared turtle (Sneary &
Lewis 2007)
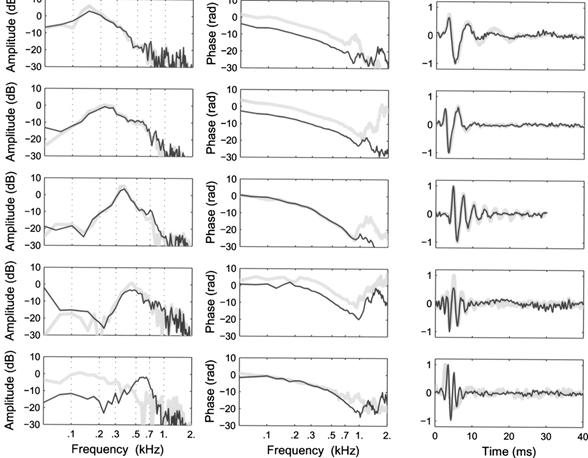
The filter functions are displayed in
the right-hand column, the amplitude and phase
components of their DFTs
are displayed in the left- and center columns, respectively.
•
In this ancient reptile, the filter functions are
compact,
•
but the band edges are not nearly as steep as they are
in the gerbil.
•
What about the American bullfrog, a relatively recent
amphibian?
d.
Sample filter-function DFTs from the American Bullfrog
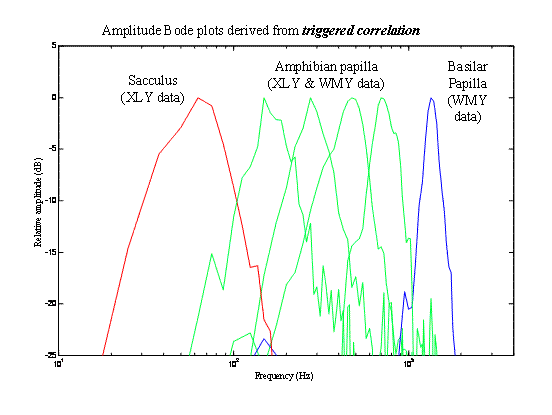
These are Bode plots (log-log plots of DFTs) of filter functions taken
by Xiaolong Yu
(XLY), Walter Yamada (WMY) and Kathy Cortopassi
(KAC).
Representative filter functions themselves (impulse responses)
are shown below and in the section (“Saccular physiology from the outside,
part I” under Sacculus). Notice that, like the mammalian cochlea, the
amphibian papilla covers its frequency
range with an array of filters, each
covering only part of the total
range. As in the cochlea, these are
distributed tonotopically
over the papillar surface-- high-frequency
sensitivity at one end, low-frequency at
the other.
•
Bullfrog band edges are very steep.
•
How might they be they achieved?
•
For the answer– we’ll do a little circuit analysis . .
We’ll consider models of
discrete processes only - - - no distributed
models of spatially-extensive wave or diffusion processes. In finite-element representations of
diffusion or waves, the behaviors of the two kinds of models converge, so we
lose no generality.
3. How Spectral Filters?
All classical physical processes can be described in
terms of non-equilibrium thermodynamics, which in turn is describable in terms
of a small set of generalized dynamic elements. In other words, with this small set of
elements, one can faithfully model the dynamics of all such processes—including
the processes that might underlie the peripheral filters of the vertebrate
ear. A broad subset of such processes
are faithfully represented by compartmental models (familiar to most
biologists), where each compartment represents a site or state in which some
entity can accumulate, and transfer between such sites or states is
accomplished by first-order kinetics—as in linear chemical kinetics, diffusion
across osmotic barriers, and heat flow.
We shall begin by determining whether or not the observed filter
properties could arise with such processes alone.
a. Compartmental models
Three-compartment model I

This represents a three-state
process (e.g. chemical reaction or diffusion) with linear kinetics.
When Jin is an
impulse at t = 0, all the internal
variables have the form …..
A e-0.20t + B e-1.56t + C e-3.25t
and the corner frequencies (rad/s) of the system are: 0.20 1.56
3.25
Three faces of A e-0.20t + B e-1.56t + C e-3.25t
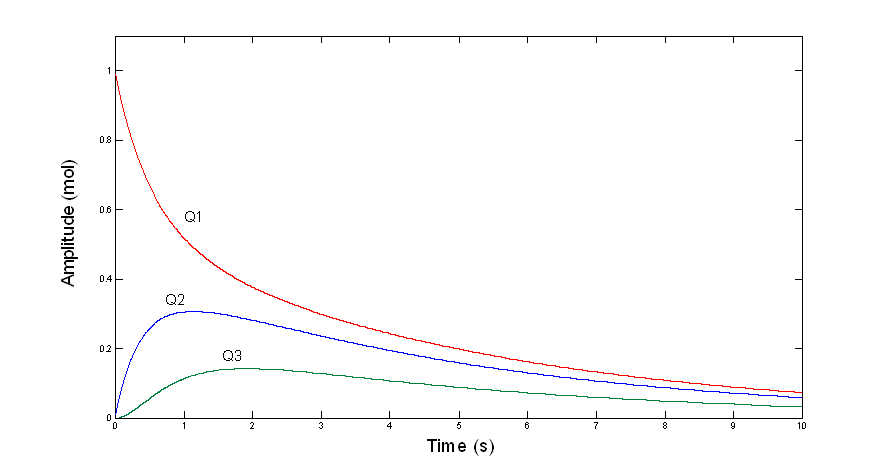
Although the functions Q1(t),
Q2(t), and Q3(t) have the same basic mathematical form,
they are distinctively different in shape—depending
(mathematically) on the magnitudes
and signs of the parameters (A,B,C) and
(physically) on the position of the compartment
relative to the input. The impulsive input fills the first
compartment instantly (in our simple
model);
the second compartment fills as the first gradually drains into it; and
the third fills
even more gradually as the second
compartment drains into it.
•
Each compartment
behaves as a leaky integrator
•
At frequencies
well below the lowest corner (0.20 rad/s), the system
is always very close to steady state (leaks dominate):
•
If Jin = K mol per s
•
then Q1 = 3K
mol
•
Q2 = 2K mol
•
and Q3 = K
mol
•
At frequencies
well above the highest corner (3.25 rad/s), the
compartments don’t have time to leak significantly during each half cycle,
•
They approximate
pure integrators,
•
and the system
approximates closely its asymptotic behavior …..
The half-cycle volume (integral) of
sinusoidal flow
decreases as the reciprocal of frequency
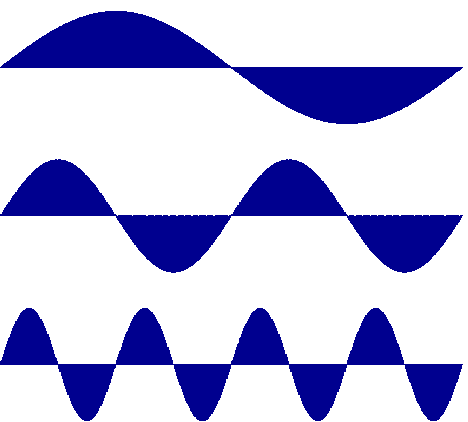
Notice that, for a sine wave of constant
amplitude, the area under each half
cycle
decreases in inverse proportion to the
frequency. In our model, positive areas
(areas above the baseline) represent
accumulation (of whatever is reacting or diffusing)
in a compartment. Negative areas (below the baseline) represent
depletion. Over
a full sinusoidal cycle the amount
accumulation equals the amount of depletion.
The
amount of accumulated stuff is maximum
each time the positive half-cycle is complete.
Asymptotic responses at high frequencies
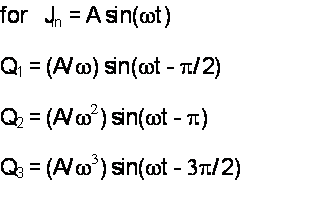
Bode plots (DFTs)
of the three faces of
A e-0.20t + B e-1.56t + C e-3.25t
The corners separating the very-low
frequency behavior (response amplitudes of all quantities
remain constant) and the very-high
frequency behavior (response amplitudes decline at slopes
that are constant on the log-log plot)
are very rounded, extending over a decade of frequency.
They are not nearly as sharp or abrupt
as those we see in the data for any of the four inner-ear
organs discussed here.
·
We can, however, make the following, very general
statements about our observations of filter properties in the ear . . . . .
Rules of thumb
•
The
Bode (DFT) plot should provide strong inference regarding the number (N) of
separate integrating processes, in cascade, between the point of observation
and the point of input.
•
The
magnitudes of the asymptotic slopes should sum to N, corresponding to 1/wN
•
This
is the same as 6N dB per octave and 20N dB per decade
•
The
range of phase change should be Np/2 radians (90N degrees)
Now we can attempt to sharpen the corners in our
amplitude Bode plots.
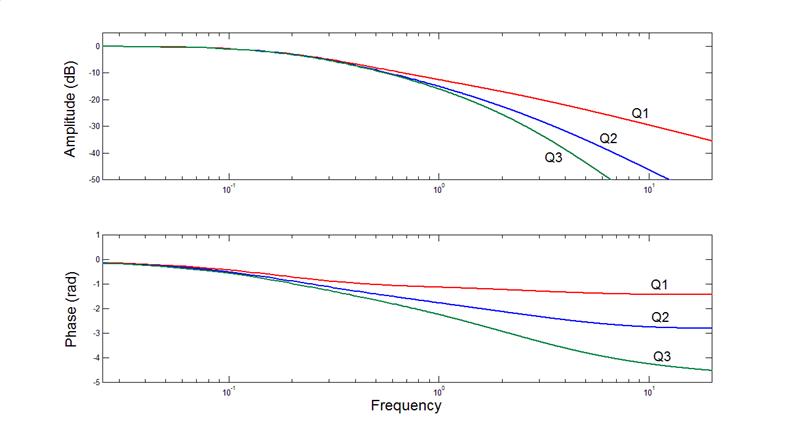
Three-compartment model II

corner frequencies (rad/s): 1.00
1.00
1.00
With its unidirectional coupling from state to state,
this three-compartment model is unrealizable, in principle, with passive nonequilibrium thermodynamic processes. Nevertheless, it
can be approached as closely as one might wish with standard chemical or diffusional kinetics. Its impulse response will reflect n
identical corner frequencies when its response is observed at the nth
compartment in the sequence. If it were
extended to six compartments (see below),
the last one in the sequence would exhibit six identical corner
frequencies of 1.0 rad/s.
Among all n-compartment models, the one with n
identical corner frequencies has the sharpest corner in its amplitude Bode
diagram (the graph of the amplitude part of its discrete Fourier transform or
DFT). It’s the best we can do with
cascades of first-order kinetics.
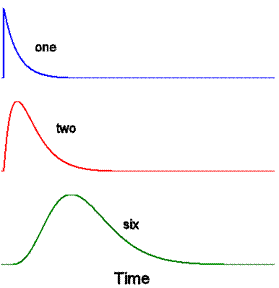
Here are impulse responses for the nth
compartment, with n being one, two and six.
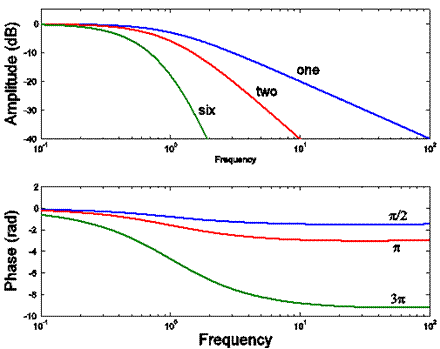
Here are Bode plots for the same three
impulse responses (graphs of the amplitude
and phase parts of their DFTs). These all are
low-pass filters.
Toward the end of this presentation we’ll see low-pass
filter functions from the bullfrog lagena. Other filter functions from the lagena are band-pass in nature, as are all of the filter
functions we have observed from the bullfrog sacculus,
AP and BP.
•
To create a band-pass filter, we must add
differentiation—
•
(in cascade
(series), anywhere along the cascade of leaky integrators).
Impulse responses of Model II with six
leaky integrators
and 0, 1, 2 & 3 stages of
differentiation
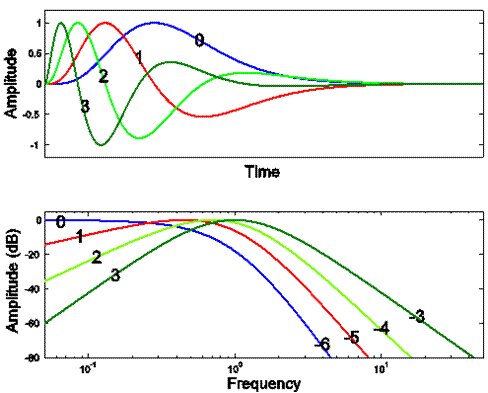
The impulse responses are plotted in the top frame,
the amplitude parts of their DFTs are plotted in the
lower frame. In the top frame, the
inserted numbers indicate the number of stages of differentiation for each
plot. In the lower frame, the numbers on
the left indicate both the number of differentiation stages and the asymptotic
slope at low frequency. The numbers on
the right indicate the asymptotic slopes at high frequency.
Our rules of thumb are augmented by one additional
statement:
•
The
slope of the low-frequency asymptote equals the number of stages of
differentiation.
The rest remain valid . . .
•
The
magnitudes of the asymptotic slopes of the amplitude Bode plots still sum to N
(the number of separate integrating processes in cascade between the point of
stimulus input and the point of observation).
•
The
phase range remains Np/2
rad.
Based on identical corner frequencies, the amplitude
DFT plots (Bode plots) shown here illustrate the sharpest filters available
with N (six in this case) first-order kinetic processes. Now we can compare this best-case result with
filter functions from the bullfrog ear.
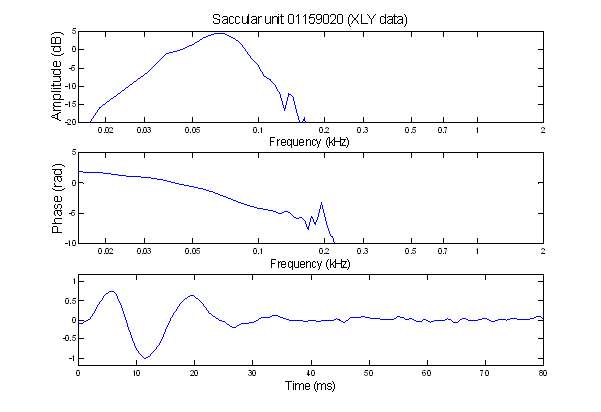
Here is the filter function from a saccular
unit (recorded by Xiaolong Yu). The impulse response is plotted in the
bottom frame; its Bode plots are above it.
The low- and high-frequency slopes of the amplitude Bode plot (top) are
3 and -5, respectively. Therefore . .
.
•
Between the
input point and the point of observation . . . .
•
the Bode plot
implies at least eight stages of leaky integration
•
with at least
three- stages of differentiation.
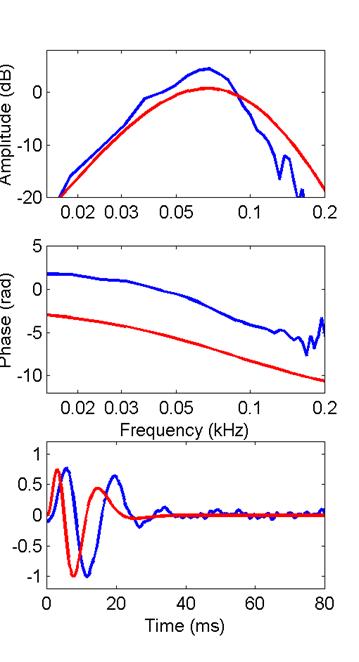
I’ll compare this bullfrog filter function with the
filter function produced by the extended model with n=8 and 3 stages of
differentiation. Then, following a
suggestion by Mark Rutherford, I’ll compare it with the model’s filter
functions with n=9 and 10. In each
case, red corresponds to the model, blue to the saccular
data.
8 stages
9 stages
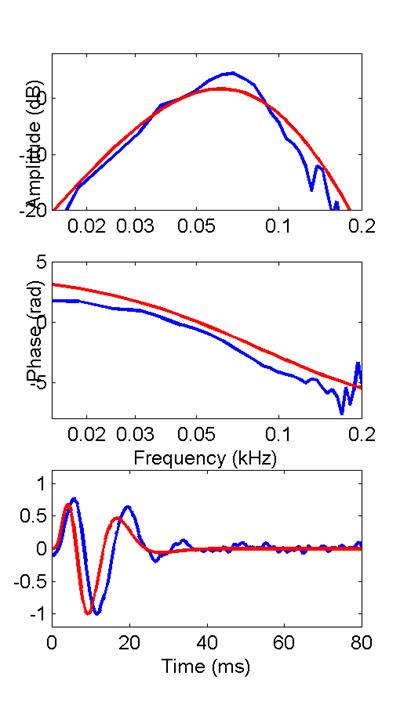
10 stages
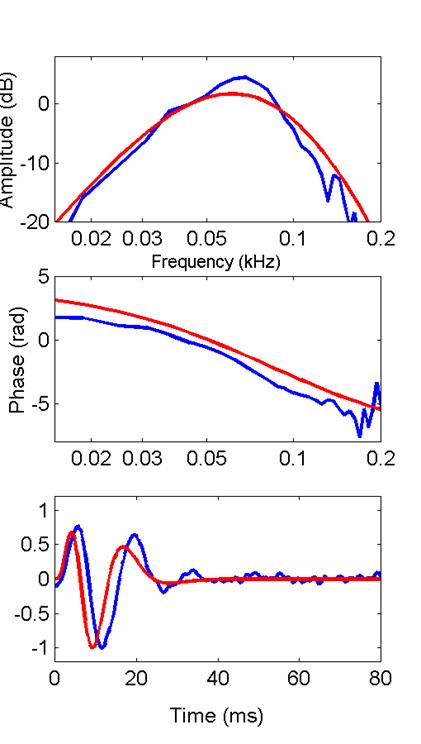
Mark was correct; we achieve a better fit to the
filter function and its Bode plot with slightly higher order. One feature that the cascade of compartments
cannot match is the near-periodicity in the filter function’s zero
crossings. Notice in the model’s
response that the zero- crossing intervals increase conspicuously as time
progresses (a feature some investigators call a frequency glide). That
generally seems not to be the case for saccular
filter responses (they don’t glide as
much). The near periodicity of the saccular filter function would lead as well to its slightly
narrower and more peaked amplitude-Bode plot.
We see the same features, to even greater degree in bullfrog AP and BP
filter functions (see below).
•
Model II gives us the narrowest tuning curve we can
get with passive compartments (leaky integrators) alone.
•
To match the bullfrog saccular,
AP and BP data we evidently need to look elsewhere.
•
Circuit theorists traditionally consider three kinds
of elements (R, L, C).
•
Compartmental models are equivalent to two-element
(R,C or R,L) circuits.
•
Now we’ll add the third element- - - giving us one
more type of response . . . under-damped resonance.
•
The impulse responses of highly under-damped
resonances are not
compact.
•
Therefore, we’ll focus on slightly under-damped
resonances.
b.
RLC circuit models
Twentieth-century circuit theory had two
complementary subfields- - circuit
(network) analysis and circuit (network) synthesis. Among the important messages one derived from
network synthesis, there is one especially appropriate to our situation
here: for any linear transfer relation
(such as our filter functions) there is an infinite number of realizations
(different ways to achieve it, physically).
Thus, no matter how well its behavior fits the data, a specific circuit
model must be considered an affirmation of the consequent. Therefore, we need not consider specific
circuit models here. All we must do is
consider the newly available response type (slightly under-damped resonance) to
see if it can compensate for the deficiencies of the compartmental model.
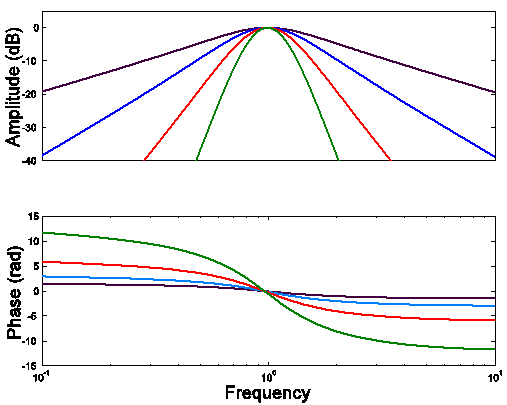
A
Filter functions for cascades of
identical, slightly
under-damped resonances
Notice that in the
higher-order filter functions, the zero-crossings are much more evenly spaced
than they were in the compartmental models.
This definitely is a step in the right direction. With appropriate
combinations of compartmental processes and slightly under-damped resonances,
we should be able to synthesize our frog inner-ear filter functions. The main point here is that we cannot do it
with compartmental processes alone, something must be added.
Here are Bode
plots for the four filter functions in figure A.
B
Note how different the Bode
plots in B are from those in C, which are for a single resonance with various degrees of damping. None of our frog filter functions yielded
amplitude Bode diagrams with concave flanks, such as those in C. The only abrupt phase shifts we found were in
a subset of saccular units at anti-resonances (as
opposed to resonances); those anti-resonances disappeared when the same saccular units were stimulated with airborne sound rather
than substrate-borne vibration.
C
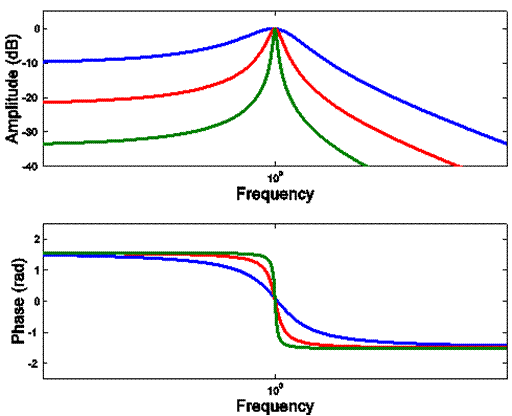
Each resonance comprises two separate integrating
processes- - one accumulating potential energy,
the other accumulating kinetic energy.
In a cascade of resonances, therefore, regardless of
the degree of damping,
•
Each
resonance in the cascade adds 2 (12 dB/oct or 40 dB/dec) to the sum of the magnitudes of the asymptotic slopes
in the Bode plot.
•
Each
resonance adds p
radians (180 deg) to the phase range.
Conclusion
to this point
With respect to band-pass filter functions from the
bullfrog sacculus, AP and BP . . . .
•
Our results, from dozens and dozens of units, suggest cascades
of slightly under-damped resonances (possibly combined with leaky
integrators).
•
Our results are
inconsistent with filters comprising single (or a few), more-highly
under-damped resonances.
•
Those are the principal strong inferences so far from
these studies of Bode plots.
•
We cannot infer how the resonances arise.
________________________________________
1.
•
They could arise from combinations of
complementary passive reactive elements analogous to L’s and C’s in electronics
(e.g., masses combined with elastic elements).
2.
•
They could arise from combinations of leaky
integrative elements (such as first-order chemical reactions and
compartmentalized diffusion processes) and feedback with active transducers
(such as gated ion channels- - - as in the electric resonance model of R.S.
Lewis & A.J. Hudspeth).
•
Which would be analogous to phase-shift
oscillators in electronics.
Note:
The Lewis/Hudspeth resonance has been strongly inferred in
bullfrog saccular
hair cells and in low-frequency hair cells from the
bullfrog amphibian papilla. This makes it the model of choice as we
attempt to account for the filter
functions associated with those
hair cells. Occam’s razor trumps affirmation of the
consequent; or,
Occam’s razor plus affirmation of the
consequent yield strong inference.
3.
•
Or, they could arise from combinations
of leaky integrative elements and Onsager’s missing passive transducer– the
anti-reciprocal transducer (or gyrator).
•
With a cascade of leaky integrators alone, signal
energy can flow only in the direction away from the input.
•
In the three cases cited above, signal energy can
bounce back and forth– flowing toward the input as well as away from it. This gives rise to the characteristic ringing
of the under-damped system.
•
The amplitude Bode plots for bullfrog saccular, AP and BP filters implies that there is some of
this back-and-forth bouncing in each of those filters.
4. Dynamic order of bullfrog filters
The dynamic order of a circuit is given by the number
of independently functioning Ls and Cs, or their equivalents, or, equivalently,
by the number of separate leaky integration processes plus twice the number of
separate resonances. For our
experimental Bode plots, it is equal to or greater than the sum of the
magnitudes of the asymptotic slopes.
For our saccular filter
function, with its dynamic order of at least eight, we infer the following:
•
Between
the input point and the point of observation . . . .
•
Bode
plot implies a cascade of n slightly
under-damped resonances plus m leaky integrators, where m +2n equals at least eight
•
with at
least three stages of differentiation.
Here is a filter function from a
bullfrog AP unit
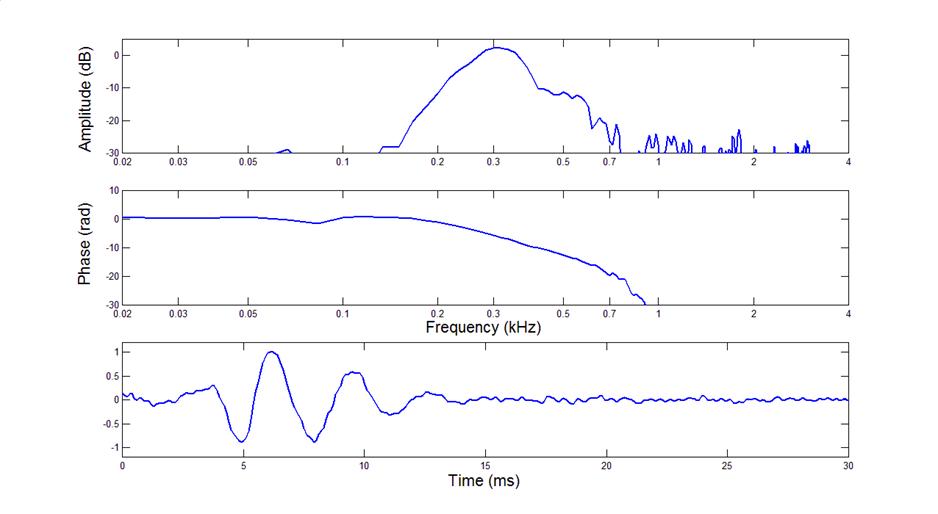
•
Between
the input point and the point of observation . . . .
•
Bode
plot implies a cascade of n slightly
under-damped resonances plus m leaky integrators, where m +2n equals at least ten
to twelve
•
with at
least six stages of differentiation.
Here is a filter function from a
bullfrog BP unit
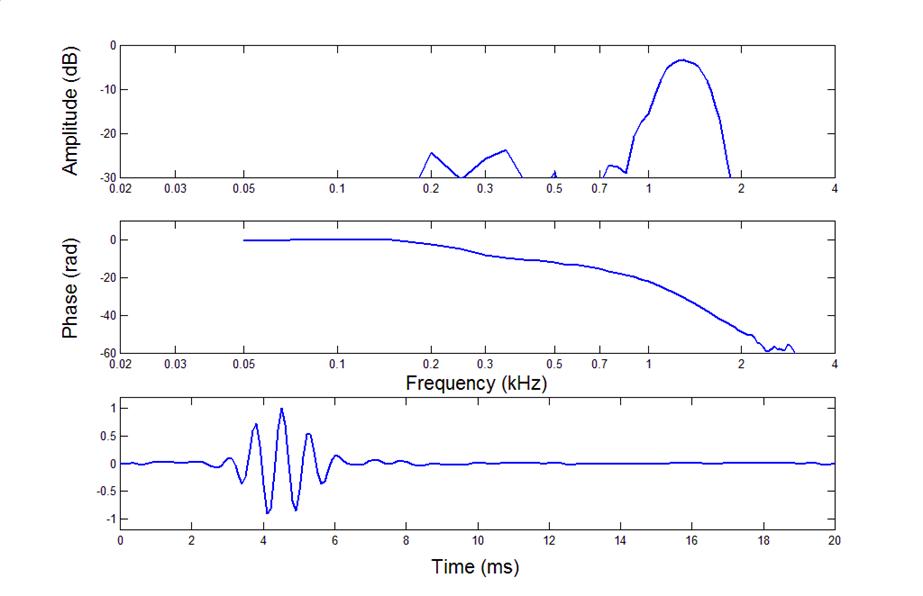
•
Between
the input point and the point of observation . . . .
•
Bode
plot implies a cascade of n slightly under-damped resonances plus m leaky
integrators, where m +2n equals more than twenty
•
with
more than ten stages of differentiation.
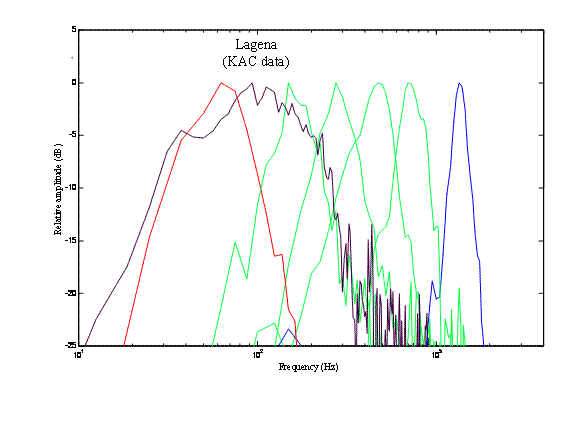
Here are filter functions from two bullfrog lagenar units
(from Kathy Cortopassi)
Here is the distribution of dynamic
order in bullfrog lagenar units,
estimated from their Bode plots
(from Kathy Cortopassi)
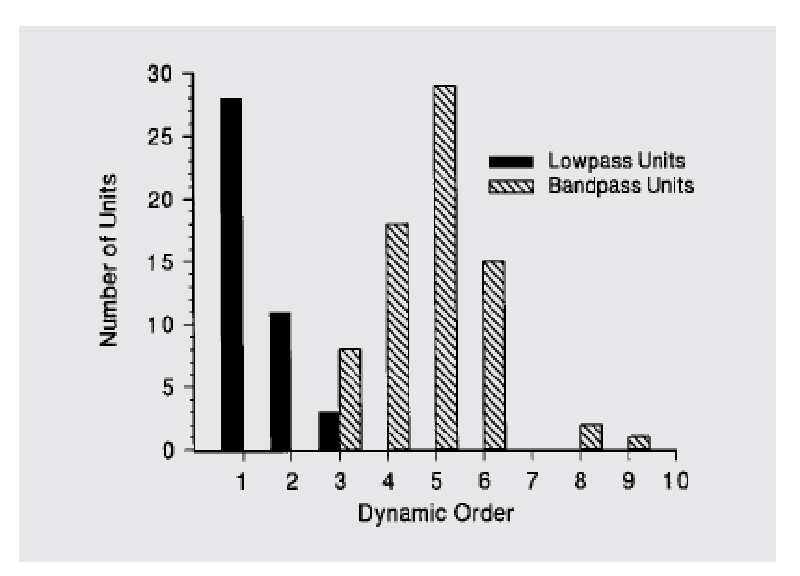
•
Between
the input point and the point of observation . . . .
•
the
Bode plots imply a cascade of n slightly under-damped resonances plus m leaky
integrators, where m +2n equals the dynamic order given in the figure.
•
Bode plots for the
low-pass filters imply no differentiation.
•
Bode plots for the
band-pass filters imply various numbers of stages of differentiation (four or
five for the Bode plot in the right-hand panel above).
.
Further Conclusions
Whether they are taken from the bullfrog sacculus, the bullfrog amphibian papilla, or the bullfrog
basilar papilla, the acoustic filter functions of the bullfrog inner ear
reflect very high dynamic order, implying large numbers of separate integrative
processes, in cascade, between the point at which stimulus is applied (external
ear canal for AP and BP) and the point at which observations are made (VIIIth-nerve afferent axon). The dynamic orders of acoustic (band-pass)
filter functions observed in the bullfrog lagena are
moderately high- - distinctly higher than those of the vestibular (low-pass)
filter functions from that same sensor.
See Cortopassi & Lewis, 1998, for further
discussion of the differences between acoustic and vestibular filter functions-
- this was the theme of Kathy Cortopassi’s doctoral dissertation.
Bottom
Line . . .
•
However they arise, the acoustic filter
functions of the bullfrog ear are beautiful . . .
•
with compact, often nearly symmetric, impulse responses
•
and very steep band edges- - -
•
these are exactly what are required for
very-high-resolution spectrographic analysis.
Footnote:
As filter functions are designed for increasingly sharp
tuning, being symmetric and compact minimizes the distortion they impose on
temporal waveforms. We can go back to
our resonant filters for examples.
Here again are the filter functions formed by cascades
of one, two, four and eight very slightly under-damped resonance (whose impulse
response is depicted in the top line).
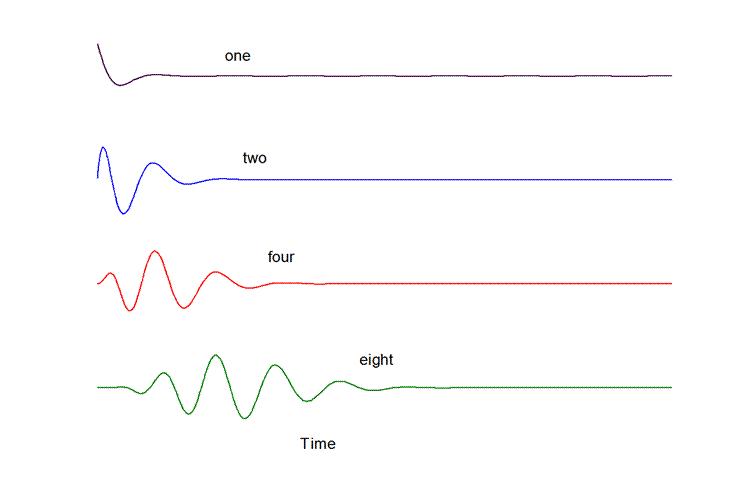
Here are the responses those filter functions would
produce when the input waveform is the stimulus depicted in the top-most line,
a tone burst at the filters’ BEF.
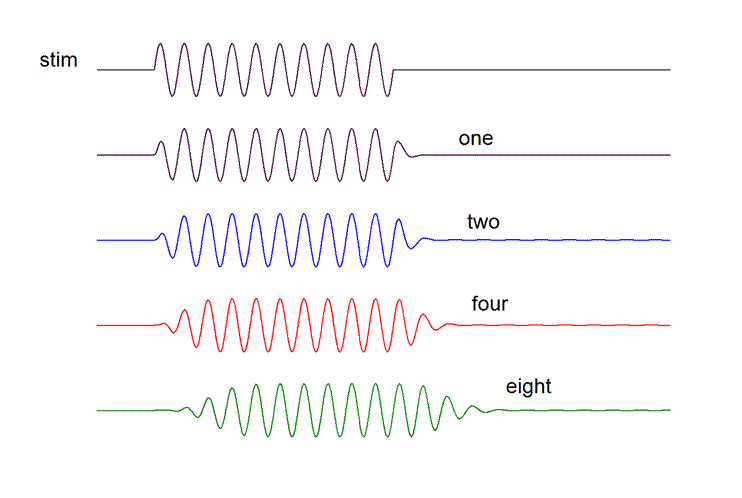
Notice that even with eight stages in
cascade (dynamic order 16)
the distortion of the envelope of the
stimulus waveform is relatively
minor, comprising largely finite rise
and fall times and slight time delay.
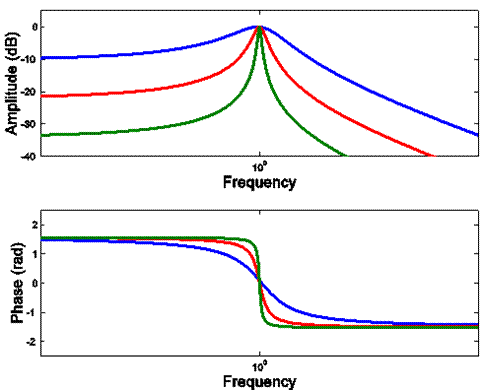
Here again are the DFTs
(Bode plots) of filter functions for a single resonance as it becomes
increasingly under-damped.
Here are the responses those filter
functions would produce when the input waveform is the
stimulus depicted in the top line, again
a tone burst at the filters’ BEF
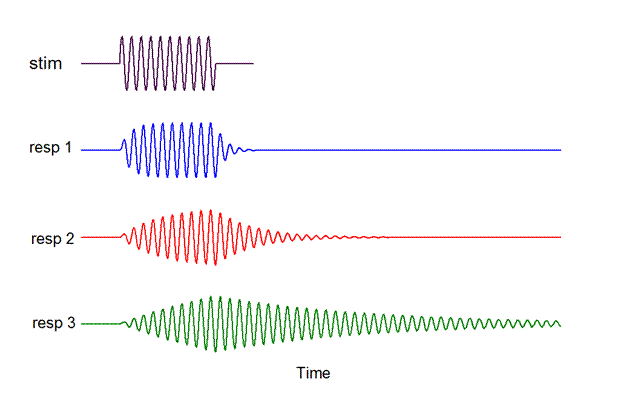
As the filter’s tuning becomes sharper,
its temporal response becomes more sluggish.
These examples illustrate the chief advantage of a
filter with high-dynamic order (many stages in cascade) over a filter
comprising a high-Q, single-stage under-damped resonance. The fact that we see the former and not the
latter in turtle, gerbil and bullfrog inner-ear acoustic senses is not
surprising.
Epilogue
As
I conclude this web page (on 03/27/2009), the biophysical circuitry underlying
the peripheral filters of the bullfrog acoustic sensors remains largely
unknown. On the other hand, the hearing
research community has become increasingly comfortable with two elements that
evidently are part of that circuitry in saccular
units and in lower-frequency units of the amphibian papilla. One is electrical resonance in the
individual (isolated) hair cells; the other is hair-bundle motility. The former has been demonstrated to include
active elements- namely gated channels; the latter may or may not be an active
process. The frequency distribution of
the electrical resonances in AP hair cells matches very well that found in
whole units. After the early
observations of the resonances in saccular hair
cells, this did not seem to be the case there.
The resonant frequencies seemed too high. More recent observations show that they do
fall into the correct range.*
It is very difficult for me to believe that
occurrence of those resonances is coincidental.
It seems that they must be participating in the tuning of the
filters. They exhibit dynamic order
two. The saccular
filters and the low-frequency AP filters exhibit dynamic orders of eight or
more. Furthermore, the range of phase
shift and the slopes of the band edges displayed in Bode plots from those
filters set a key constraint on the underlying biophysical circuitry—the eight
or more integration processes implied by those high dynamic orders must be in
cascade between the point of stimulus input and the point of spike triggering. In other words, parallel connection by a
nerve fiber to four resonant hair cells will not suffice. If filtering in sacculus
and low-frequency part of the AP depend solely on those resonances, then the
resonances must be connected in cascade.
What would be needed for that sort of connection is reverse transduction
in the hair cells (hair-bundle motility driven by the electrical
resonance). This was clear even before
reverse transduction had been observed in non-mammalian vertebrates (Lewis,
1987). In the earlier discussions of
reverse transduction, it was proposed as a device to reduce damping in the
cochlea. In the bullfrog, it was and is
needed for something very different; it is needed to link resonances into a
cascade chain—thus providing peripheral filters with spectral resolution made
high by steep band edges rather than sharp resonances.
That
sounds simple. We now have the
resonances, and we now have the reverse transduction. But how do we link them? Suppose we had a cascade chain of four hair
cells, with input to the first one, output from the last one. That would give us the sort of Bode plots
we’ve observed in the actual units. But
how is the input limited to the first hair cell and the output limited to the
last? Nothing seen so far in the saccular or AP micro-morphologies suggests answers to that
question.
Considering mechanical elements only, which seems to be the entire story
in higher-frequency AP units, Ellen Leverenz and I
addressed the same sort of question in 1983.
We couldn’t answer it then, and I don’t see an answer to it now. But the answer must be there, someplace. Considering the AP tonotopy
we had just found, along with AP frequency-threshold tuning curves we obtained
while doing that, we had surmised that AP tuning (filtering) was accomplished
by some sort of traveling-wave process.
What we were looking for in the AP micro-morphology, and did not find,
was micromechanical underpinning for such a process. We were left with the conjecture that it
somehow resided in the tectorial membrane.
*I thank Mark Rutherford for pointing this
out to me.