The Bullfrog Sacculus
from the Outside
Part 2
Inferences about saccular signal processing and its
underlying mechanisms
Now that we have compared the general
signal-processing properties of the bullfrog sacculus
to those of the other three acoustic sensors in the bullfrog ear, we shall move
on to the sacculus itself, in more detail. Before we do that, here is one more
comparison between the sacculus and its spectral
neighbor, the bullfrog amphibian papilla (AP).
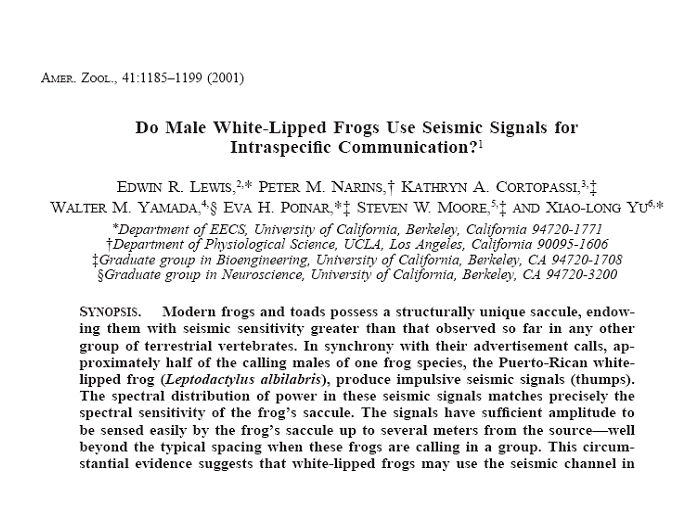
As we saw in Part I,
the ac response component in the bullfrog amphibian papilla is accompanied by a
negative dc adaptation component, with the ac component growing linearly with
stimulus amplitude, the dc component growing as the square of the stimulus
envelope. Here, on the left we see the
impact of that combination on a representative series of cycle histograms taken
under steady-state conditions (the sinusoidal stimulus was applied for several
seconds before the data were collected).
For low-level auditory stimuli, the peak ac response was relatively
strong and grew with increasing stimulus amplitude. For higher-level stimuli, the negative dc
response began to overwhelm the ac response, although the phase of the peak of
the latter remained fixed. For stimuli
equal to or greater than 0.7 Pa, no steady-state response at all could be
observed in this unit. There remained,
however, a brief (transient) response when the stimulus was first applied.
Bullfrog saccular
units showed no such dc response. The ac
response continued to grow (linearly, as we shall soon see), but the phase of the peak steady-state
response shifted leftward as the stimulus amplitude increased.
These effects are modeled in the following
two figures.
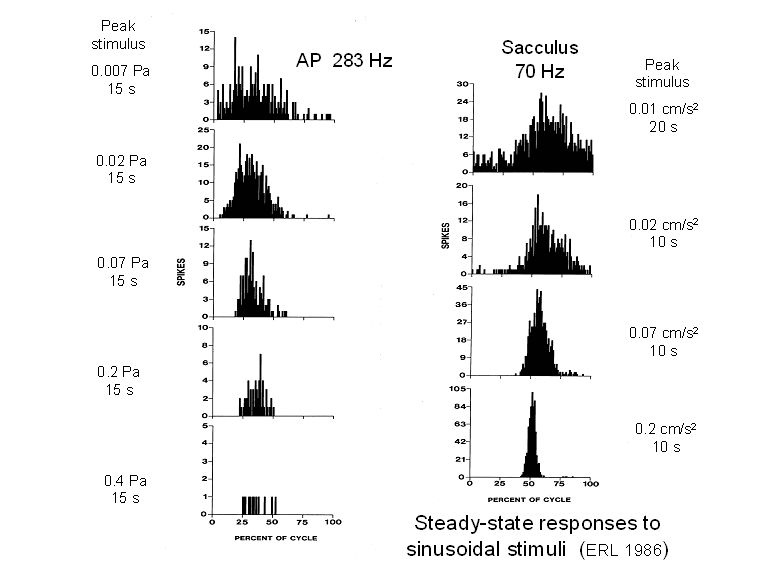
Imagine that the blue line is the generator
potential at the spike trigger of the unit being observed, and that the
generator potential comprises an inherent (resting) dc level (rst bias) combined with the ac and dc responses to the
stimulus. The total resulting dc level (rsp bias) thus is the sum of the resting bias and the dc
response. Between the upper and lower thresholds (thr)
in this model is the region of continuous spike-rate modulation, As long as the
generator potential falls in this region, it produces continuous modulation of
instantaneous spike rate. When it moves
below the lower threshold, the instantaneous spike rate is zero; when it moves
above the upper threshold, the instantaneous spike rate is at its maximum
level. For the stimulus frequencies
employed in the cycle histograms of the previous figure, the unit produced at
most one spike per cycle (owing to refractoriness). Thus the dynamics of the spike trigger play
an important role in the actual timing of the spikes. The model does not explicitly include that
role.

Apply the same assumptions to this
model. Here, however, the dc bias
remains fixed at its inherent level. As
the amplitude of the generator potential sweeps beyond the upper and lower
thresholds, spike trigger dynamics guarantee that spike occurrences will be
limited to the rising phase of the generator potential, as it passes through
the region of continuous modulation. As
the duration of that phase is shortened, spike occurrences will be confined to
a shorter time interval, leading to a higher peak instantaneous spike
rate. This model accounts for the
leftward shift in the peak as well as its continuing
to increase with increasing stimulus amplitude— even with very high-level
stimuli (see next figure).
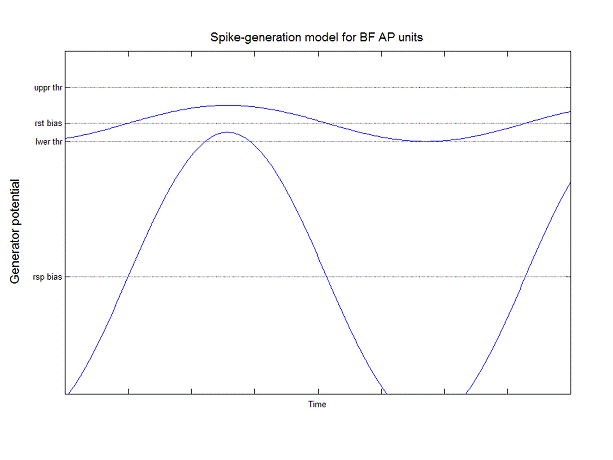
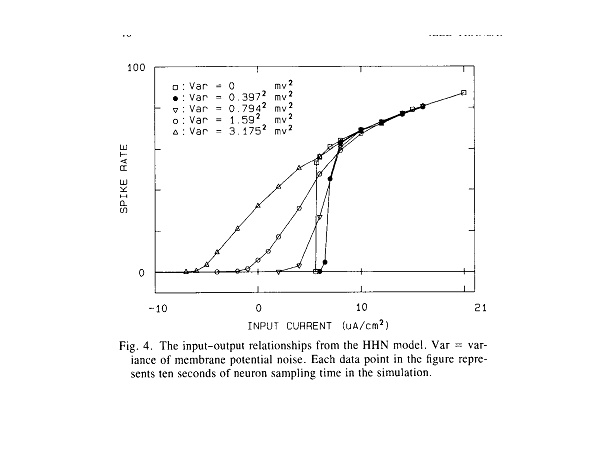
The figure caption here was taken from
Lewis et al., 2001. According to the
model, when the peak (instantaneous) spike rate response exceeds the mean spike
rate, the spikes are confined to the rising phase of the generator potential as
it sweeps through the region of continuous modulation. Truly linear response (through all phases of
the sinusoidal generator potential) occurs when the peak response is well below
the mean spike rate. The ordinates of
the circles were computed by taking the discrete Fourier transform of the cycle
histogram and computing the peak amplitude of the fundamental (50 Hz in this
case). The ordinates of the squares were
computed by observing the peak spike rate directly in the cycle histogram, and
subtracting from it the mean spike rate of the unit at rest (unstimulated).
Xiaolong Yu combined noise
with the Hodgkin-Huxley model to demonstrate how dithering of a spike trigger
might produce a region of continuous modulation of instantaneous spike
rate. French and Stein had done the same
thing twenty years earlier with simpler triggers (e.g., integrate-and-fire
models). We chose to extend their
studies to the Hodgkin-Huxley model because that model includes both refractory
mechanisms (whereby threshold is increased temporarily following a spike) and
accommodative mechanisms (whereby threshold tends to move away from a slowly
increasing generator potential). We were
interested in how these phenomena, together, would shape the cycle histograms. We also were interested in the biophysical
feasibility of the noise amplitude required for effective dithering.
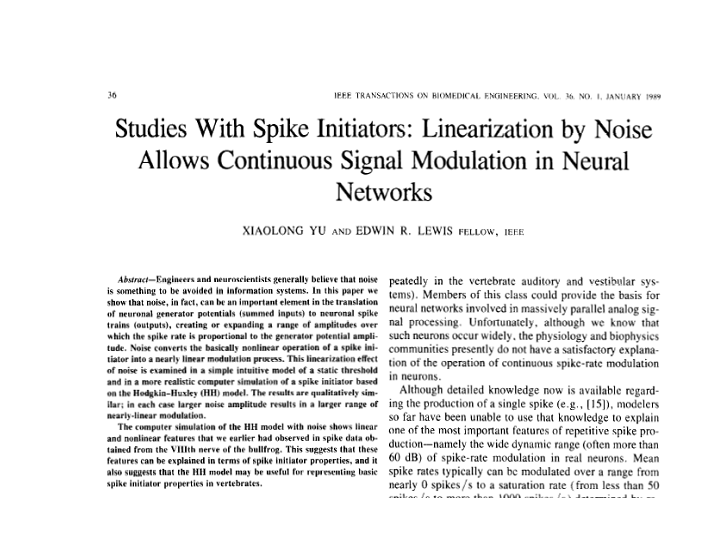
This figure illustrates the effects of
dithering noise at very low levels.
Without noise added, the Hodgkin-Huxley model responds to a
continuously-increasing stimulus current by jumping suddenly from
0 spikes/sec to near maximum spike rate.
Dithering noise eliminates that jump and provides a broad region of
continuous spike-rate modulation. The
following two figures compare responses of a saccular
unit to four levels of sinusoidal stimulus amplitude to the responses from the
dithered Hodgkin-Huxley model (HHN model) to four levels of sinusoidal
generator-current amplitude. It seems
that here we have a reasonable biophysical basis for the two graphical models
with their continuous spike-rate modulation regions.

Here are linear gains taken from our early
work on the seismic sense of the bullfrog sacculus.

These gains were computed for saccular units by discrete Fourier transform of responses
to low-amplitude vibrational sinusoids. Note that at the threshold of the previous
champs (snakes and cockroaches), which is 0.02 cm/sec2, units with
gains in the 1000-3000 bracket would produce peak responses of 20 to 60 spikes
per sec. In many cases, that would be
beyond the linear operating ranges of the units.
And here
are best (band-center) tuning frequencies.
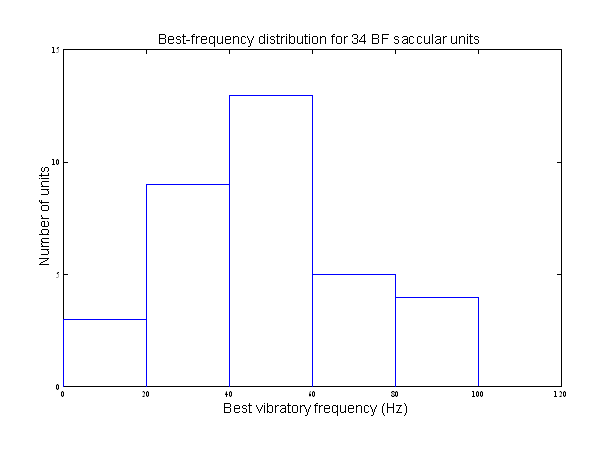
These data were taken by Ellen Leverenz, Hironori Koyama, Richard Baird, and me in the
early 1980s. The distribution of best
frequencies conforms well to what we have observed in the years since that
time. It is useful to note that the
frequencies of the electrical resonances observed in bullfrog saccular hairs cells ranged from about 90 Hz to about 250
Hz—mostly well beyond the range of saccular sensitivity.
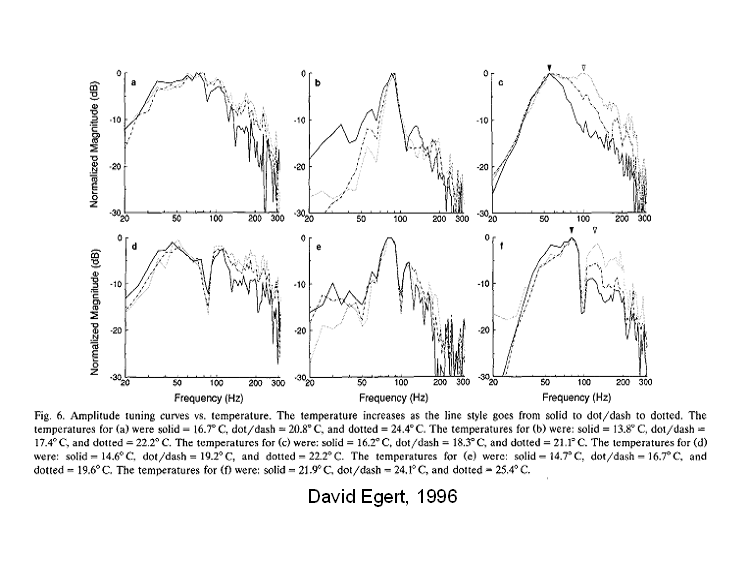
To test for
involvement of electrical resonances in bullfrog saccular
units, David Egert examined the temperature
sensitivity of tuning. Electrical
resonances in hair cells are strongly dependent on temperature. David found virtually no variation in the
frequencies of tuning peaks and troughs as the temperature was altered. This result strongly implies that the
resonances, observed in isolated hair cells, are not involved significantly in
the tuning of saccular sensitivity.
Aside
from the deep, persistent notches, the ragged nature of these tuning curves is
a consequence of short sampling times used in the triggered-correlations.
The following figure shows results of
similar experiments (by Pim van Dijk)
on the bullfrog amphibian papilla.

In that organ, tuning peaks of
lower-frequency units units shift markedly with
temperature changes, implying strong involvement of electrical resonances.

Bullfrog saccular
units respond to airborne sound (presented to the tympanum) as well as to
substrate vibration. Xiaolong
Yu found that there frequently were conspicuous differences between the
auditory and vibrational tuning curves of bullfrog saccular units. The
latter often exhibited deep anti-resonant notches (for which David Egert subsequently found no temperature dependence). This implies that auditory and vibrational stimuli take different routes to reach saccular hair cells.

Here are the two traditionally-cited
pathways for acoustic stimuli to reach the sacculus. Both paths converge at the oval window. The anti-resonance notches were present in
some saccular units, absent in others— in any given
bullfrog. Furthermore, where they
occurred, their frequencies varied from unit to unit in any given
bullfrog. Therefore, their source did
not lie in a common signal path from the periphery. This suggests profound differences in the sources
of auditory and vibrational sensitivities in the
bullfrog sacculus.
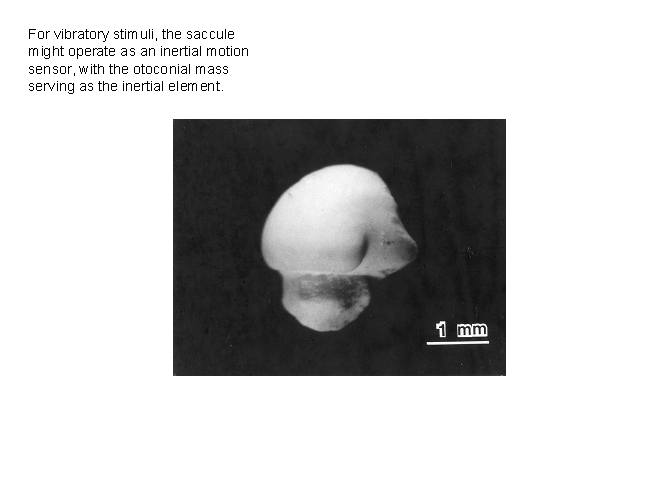
The investment in the bullfrog saccular
otoconial mass, shown here in a dry state, is huge. When one opens the otic
capsule of the frog, the saccular otoconial
mass is by far the largest visible structure.
One supposes that it must participate importantly in saccular
vibratory sensitivity—perhaps as a sensing mass that tends to stay put as the
rest of the ear is subjected to vibration, allowing the sacculus
to function as an accelerometer. The otoconial mass actually is a viscous
slurry of otoconia (calcium carbonate crystals) in a
gel-like medium, surrounded by an inextensible but flexible membrane. It is
connected through a gelatinous pad to individual hair bundles. It is easy to imagine variations in tuning
properties, including the occurrences of anti-resonances, from place to place
over that pad. On the other hand, it is
not clear why those anti-resonances would not come into play for acoustic
stimuli propagating from the tympanum, through the oval window to the sacculus. It is
interesting to contemplate the micromechanical differences between otoconial motion that would be induced by whole-body
acceleration and those that would be induced by differential motion of the oval
window alone.
This takes us to the inside of the bullfrog
ear—beyond the scope of this presentation.
Our goal, in the research presented here, was to examine the bullfrog
ear with minimal disturbance to its internal mechanical milieu. Of course our interest in the bullfrog saccule extended both inward-- into that milieu, and
outward—to the role of vibration in frog behavior.

We studied micro-morphology in the amphibian ear.

We identified correspondences between micro-morphological structures and
function.
We examined the morphogenesis of the
frog sacculus and its hair bundles.

We discovered how amazingly sensitive the frog saccule
was to vibrational stimuli .

And, together with Peter Narins,
we found evidence that frogs can use vibrational
signals for communication—
a useful acoustic
alternative when the airborne pathway is cluttered with noise and interference.