Discussion
It seems clear that various neobatrachian radiations were accompanied by conspicuous
changes in AP morphology, especially in the length of the caudal extension and
the proportion of total-AP hair cells contained in it. The degree to which AP complexity and
plasticity may or may not have accelerated that radiation has been discussed
(CL Richards-Zawacki, J. Evol. Biol. 19: 1222-1230; MJ Ryan, Proc. Nat. Acad. Sci. 83:1379-1323). A central issue is the role, if any, of the
AP in discrimination of advertisement calls, and thus its potential role in
enhancing reproductive isolation. It
seems almost universal among anurans to be the basilar papilla (BP) that is
tuned to the dominant spectral component of a frog’s call. And that component typically is well above
the top of the AP tuning range. As I
have pointed out elsewhere, I consider the tuning properties of the bullfrog BP
to be amazing—the sort of properties a master electrical engineer might design
to yield a combination of superb spectral resolution and superb temporal
resolution. That is a combination
requiring, among other things, high dynamic order—i.e., a very large number of
independent integrative processes between the point at which the stimulus
enters the ear and the point at which VIIIth-nerve
spike trains are generated (see Tuning in the bullfrog ear). These tuning properties were revealed by our
use and analysis of 2nd-order Wiener kernels.
With its broad sprectral
range and tonotopically distributed BEFs, the anuran AP seems very much like a general auditory
receptor—analogous to the reptilian and avian basilar papilla and the mammalian
cochlea. There are instances in which a
frog’s advertisement call has spectral components in the AP range—which is
usually well below the BP range. In our
1992 paper, for example, Eva Hecht, Peter Narins and
I showed that the advertisement calls of six of the eight Puerto-Rican Eleutherodactylus
species we studied had substantial energy in the spectral range of the AP. In three of the eight species, there were
substantial spectro-temporal components at
frequencies that would excite even the low-frequency (rostral)
end of the bullfrog AP. Capranica found the same thing true in the bullfrog. The advertisement call of our other Puerto
Rican friend, Leptodactylus albilabris, also
has an AP component. The early part of
its call has periodicity close to 1100 Hz; the later part is close to 2200
Hz. Each call must bing the caudal end of the AP, then bang the BP. The paddle-shaped expansion of the caudal
end of the AP (see SEM under “mid-branch Hyloides”)
might be an adaptation to enhance signal to noise ratio in detection of the bing
part. Hyla cinerea also has a bing-bang call, but the two pieces are simultaneous (H. Carl Gerhardt, J. Exp. Biol. 74, 59-73 (1978)). Its AP
also exhibits a paddle-shaped end of its caudal extension, just where the bing
part should strike (again, see SEM in “mid-branch Hyloides”). Before we attribute too much to the presence
of the paddle, I should note here that in a species (Bufo americanus) with exceptionally variable
AP shape, one specimen exhibited a paddle-shaped end to its caudal
extension. As far as I know, the trill
of the American toad plays no notes at all on the AP scale.
Two of our Eleutherodactylus species had calls
that would not excite the AP at all, and the same thing seems to be true of
many anuran species (see the Richards-Zawacki paper cited above). Perhaps the AP is shaped more frequently by
the frog’s evolving micro- and macro-habitats than by its evolving
vocalization. Over and over again, as
Richards-Zawacki points out, the evolution of the
advertisement call and the evolution of the BP must have been very tightly
coupled. The BP plasticity implied by
this is especially remarkable—given the apparent complexity of BP tuning
mechanisms.
Here is Figure 4
from Corrine Richards-Zawacki’s paper
(all of the families in the figure are from
the Hyloides and Ranoides limbs)
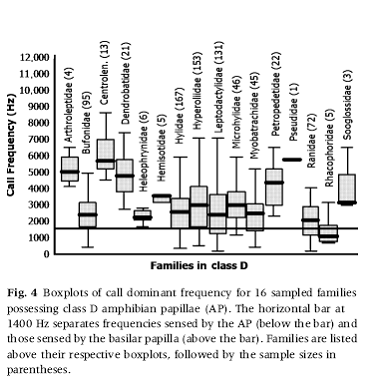
Among our eight Eleutherodactylus species, four (E. portoricensis,
E. hedricki, E. wightmanae and E. richmondi)
are strictly mountain rain-forest species, one (E. coqui) is found everywhere, including the mountain rain
forests, one (E. cooki)
lives in caves formed by piles of very large boulders, and two (E. antillensis and
E. brittoni)
are found largely at lower elevations.
The microhabitats for calling males in three (E. portoricensis, E. wightmanae
and E. richmondi)
of the five mountain species are very similar—all call from the ground or close
to it. Among these three species we
find the proportion of total AP hair cells in the caudal extension ranging from
slightly above 0.5 (in E. wightmanae) to slightly above 0.6 (in E. richmondi). The call of E. wightmanae has no AP spectral
component whatsoever. That of E. richmondi has strong components
falling in the middle of the AP range; and E.
portoricensis
has weak components at the upper end of the range.
If we consider presence of a caudal
extension with a large proportion of total the AP hairs to be a derived trait,
then in E. richmondi
and E. coqui it would be highly derived. The same thing would be true of Leptodactylus albilabris. In the anurans on the terminal branches of
the Hyloides limb, the bufonids,
the trait would be considered more primitive (in some even more primitive than
in our sooglossid (Thomasette’s frog)). This suggests that along the limb itself the
trait might have remained in its primitive state, allowing it to be shaped
more-or-less independently in the evolution (radiation) of each branch.